Car T Therapy Singapore
Novartis Gilead and BMS are the top CAR-T therapy companies in APAC. The immune system is made up of a variety of cells and organs that normally protect the body from infection and cancer.

Car T Cell Therapy Sino Biological
As these treatments gain regulatory approvals and become an available option to patients meeting disease indications administrative and logistical challenges in delivering CAR.

. Kymriah approval in Singapore is the third approval of a CAR-T therapy this year in APAC. Supported by a growing investment flowing into CAR-T research and landmark approvals of the CAR-T cell therapies Kymriah Yescarta Tecartus Breyanzi and Abecma CAR-T companies are on the rise. While first generation chimeric antigen receptor T CAR-T cell therapy has shown impressive results curing blood cancers at significantly higher rates than previous therapies issues such as high costs complexity of treatment and potential life threatening side effects have limited their use.
Global manufacturing of CAR T cell therapy. Critically the reimbursement pathway enabling patient access has been successfully navigated in Australia for CAR-T and ultimately opened the channel for funding of other cell therapies. Practical Considerations in Delivering CAR-T Cell Therapies in Singapore.
After a few weeks you have a drip containing these cells back into your bloodstream. But the fact that the first approval is for a therapy for children and. CAR T-cell therapy is a very complex and specialist treatment.
Presented by Singapore Economic Development Board SGInnovate Biotech Connection Singapore Partnered with Enterprise Singapore Accelerate. To answer the question of whether they are worth the high costs of treatment I think we need to keep in mind that these products have induced durable remissions in 35 to 40 of this pretreated. CAR T-cell therapy is particularly effective for patients diagnosed with Myeloma aggressive forms of ALL Acute Lymphoblastic Leukaemia and high-grade NHL Non-Hodgkin Lymphoma.
Singapore 9 March 2021 Novartis announced today that the Health Sciences Authority HSA has approved Kymriah tisagenlecleucel as the first commercial chimeric antigen receptor T-cell CAR-T therapy in Singapore under the new cell tissue and gene therapy products CTGTP regulatory framework. Tessa Therapeutics will open a plant in Singapore for its CAR-T programs while CellVec has opened a viral vector facility on the island state to support customers gene therapy projects. The previous treatments in this patient population include.
An important component of the immune system are T-cells which have the capacity to hunt down and destroy abnormal. Dr Chen Yunxin Consultant Department of Haematology Singapore General. The available CAR T-cell therapies are approved for use in the third-line setting when disease has relapsed following AHCT or fails to respond to second-line salvage therapy.
Levine BL Miskin J Wonnacott K Keir C. The progress made with CAR T-cell therapy in children with ALL has been fantastic said Terry Fry MD a lead investigator on several POB trials of CAR T cells who is now at Childrens Hospital Colorado. Methods Clinical Development.
Kymriah will be the first CAR-T therapy to be approved in Southeast Asia. With this treatment a specialist collects and makes a small change to your T cells. CAR T-cell therapy is a type of immunotherapy.
Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. A five-year-old boy with leukaemia is to go to Singapore for further treatment which his parents hope will save his life. Tisagenlecleucel is the first therapy to be approved by the Singapore Health Sciences Authority under the Cell Tissue and Gene Therapy Product Regulations enacted on 1 March 2021.
You might also hear it called a type of adoptive cell transfer. Of CAR-T at the Peter MacCallum Cancer Centre ensuring that all stages of the CAR-T pathway are able to remain in Australia. Kymriah was approved in Australia in December 2018 and in Japan in March 2019.
Chimeric Antigen Receptor CAR T-cell therapy is changing the treatment landscape of haematological malignancies. On 4 March 2021 Novartis organized a launch symposium for tisagenlecleucel a CD19 chimeric antigen receptor CAR T-cell therapy approved for the treatment of paediatric. Join us on the 21st of this month as we explore the exciting field of cell therapies including CAR-T and TCR-T cell therapies which display great potential to transform cancer treatment.
CAR T-cell therapy is a new form of immunotherapy that uses specially altered T-cells to directly and precisely target cancer cells. Makita S Yoshimura K Tobinai K. SGH is the first Kymriah treatment centre in Southeast Asia to become operational.
Next generation CAR-T cell therapy made using pluripotent stem cells. Oscar would be part of a trial of CAR-T therapy. Are one form of immunotherapy used to treat advanced cancer.
Relapse of non-Hodgkin lymphoma such as diffuse large B-cell lymphoma can be managed with CAR T-cell therapy especially when at least two prior treatment regimens have failed to. Explaining how the therapy works Professor William Hwang medical director of the National Cancer Centre Singapore said. Swiss pharmaceutical company Novartis said that it is the first commercially approved CAR-T therapy in Singapore.
In this webinar Dr Chen Yunxin will share insights from her time at the Memorial Sloan Kettering Cancer Center a pioneering and renowned institute in the delivery of CAR T-cell therapy and how this therapy can be applied to and implemented in the local context. Kymriah a CD19-directed genetically modified autologous T-cell. In the field of adoptive therapy CD19-targeted chimeric antigen receptor CAR T cells have yielded remarkable clinical success in certain types of B-cell malignancies and substantial efforts aimed at translating this success to myeloid malignancies are currently underway.
Singapore-based biotech Tessa is developing its own autologous cellular therapies and has told us it plans to open a 90000 square-foot plant to support. Conventional treatment for cancer usually involves one or a combination of. 56 minutes agoThe Food and Drug Administration FDA approved the CAR T-cell therapy Carvykti cilta-cel for the treatment of adults with relapsedrefractory multiple myeloma who underwent four or more previous treatments according to Janssen and Legend Biotechs the manufacturers of the therapy.
In CAR-T cell therapy a patients immune cells produced in the body are programmed in the laboratory to become cancer cell killers. CD19-targeted CAR T cells were initially tested in adults. CAR T-cell therapy is an approach that is being explored as an alternative to conventional treatments for several forms of cancer.
Continued from previous page. Prof Dario Campana is one of two pioneer researchers in CAR-T cell therapy who successfully treated children and adults with Acute Lymphoblastic Leukemia ALL recently at National University Hospital NUH Singapore. Dr Toh Han Chong Senior Consultant Division of Medical Oncology and Deputy Director National Cancer Centre Singapore NCCS a member of the SingHealth group shares more on the new cancer therapy.
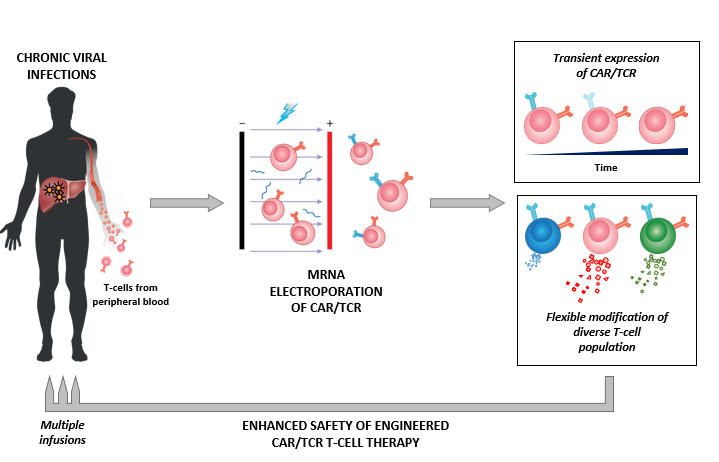
Duke Nus Scientists Explore Using Own Immune Cells To Target Infectious Diseases Including Covid 19

Autologous Car T Cell Manufacturing Using A Semiautomatic Closed Modular Workflow Bioprocess Internationalbioprocess International

Car Nk Cells A Promising Cellular Immunotherapy For Cancer Ebiomedicine

Global Manufacturing Of Car T Cell Therapy Molecular Therapy Methods Clinical Development

Car T Cell Therapy Market Size Growth Industry Report 2020 2026
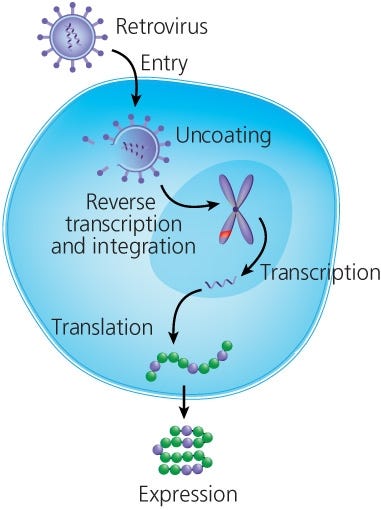
Overview T Cell Therapy Cell Therapy Research Areas Of Interest Scientific Resources

Global Manufacturing Of Car T Cell Therapy Molecular Therapy Methods Clinical Development

Global Manufacturing Of Car T Cell Therapy Molecular Therapy Methods Clinical Development

Car Nk Cells A Promising Cellular Immunotherapy For Cancer Ebiomedicine
Komentar
Posting Komentar